GDPR Compliance for Cross-Border Patient Platforms

Key Takeaways
GDPR Compliance for Cross-Border Patient Platforms
GDPR compliance is non-negotiable for digital health platforms handling EU patient data, even if the platform operates outside the EU. Here’s what you need to know:
- Applicability: GDPR applies to any platform processing personal data of EU residents, regardless of location.
- Health Data Rules: Health data is classified as a sensitive category, requiring explicit patient consent and robust security measures like encryption.
- Data Transfers: Transfers outside the EU need safeguards like Adequacy Decisions, Standard Contractual Clauses (SCCs), or Binding Corporate Rules (BCRs). U.S. companies can also use the EU-U.S. Data Privacy Framework.
- GDPR vs. HIPAA: GDPR has stricter consent and breach notification requirements compared to HIPAA, with fines up to €20M or 4% of global revenue.
- Best Practices: Platforms should map data flows, implement encryption, and provide tools for patient rights like data access and deletion.
Failure to comply can result in severe penalties and loss of patient trust. Platforms like PatientPartner simplify compliance by integrating GDPR safeguards into their operations.
Core GDPR Requirements for Patient Platforms
When GDPR Applies to Cross-Border Platforms
The General Data Protection Regulation (GDPR) doesn't just apply within the borders of the European Union. According to Article 3, it also applies to platforms outside the EU if they process the personal data of EU residents. This can happen in two key scenarios: when a platform offers services to EU residents or monitors their behavior. For patient platforms, even indirect targeting of EU patients triggers GDPR compliance. Indicators include offering services in EU languages (like German, French, or Italian), accepting euros as payment, or using tools that track EU users’ health metrics or online activities.
As Ben Wolford, Editor in Chief at GDPR.eu, explains:
"The law, therefore, applies to organizations that handle such data whether they are EU-based organizations or not, known as 'extra-territorial effect.'"
For instance, a telemedicine platform based in the U.S. that allows German patients to book appointments must comply with GDPR, regardless of the server's location. Similarly, using cookies or IP address tracking to monitor patient engagement within the EU places your platform under GDPR's jurisdiction. If your platform processes health data on a large scale, you may also need to designate an EU representative under Article 27. This representative acts as a point of contact for both supervisory authorities and data subjects.
Next, let’s look at how GDPR handles health data, emphasizing the role of explicit consent and other safeguards.
Health Data and Explicit Consent Requirements
Given GDPR's broad reach, handling health data comes with extra responsibilities. Under Article 9, health data is classified as a "special category" of personal data. Processing this type of data is generally prohibited unless certain conditions are met. The most common and preferred approach is obtaining explicit consent from patients, as outlined in Article 9(2)(a).
Explicit consent means a clear and specific agreement, often provided through a written statement or verified electronic confirmation. Importantly, pre-ticked boxes or implied consent do not meet the standard. Consent must be freely given, informed, and unambiguous, with a clear explanation of the purpose for processing the data.
Platforms must satisfy two requirements: establish a lawful basis under Article 6 (such as consent or a contract) and meet the specific condition under Article 9 for processing sensitive health data with explicit consent.
The table below outlines key conditions for processing health data:
| Condition | Description |
|---|---|
| Explicit Consent | The individual has provided clear and specific permission for the processing. |
| Vital Interests | Processing is necessary to protect a life when the individual cannot provide consent. |
| Health or Social Care | Necessary for medical diagnosis, treatment, or health system management under professional secrecy. |
| Public Health | Necessary for public interest reasons, such as addressing cross-border health threats. |
If your platform uses health data for automated decision-making, like AI-driven treatment suggestions, Article 22 mandates explicit consent before proceeding. Additionally, if health data is processed on a large scale or used to determine access to services, a Data Protection Impact Assessment (DPIA) is required.
sbb-itb-8f61039
Cross-Border Data Transfers Under GDPR
Approved Data Transfer Methods
Once you've confirmed that GDPR applies and obtained explicit consent, the next hurdle is managing international data transfers. While GDPR doesn't outright ban these transfers, it does demand specific legal safeguards to ensure patient data stays protected when leaving the EU.
Start by checking for an Adequacy Decision under Article 45. The European Commission has named 15 jurisdictions - like the UK, Switzerland, Japan, and the U.S. (for organizations certified under the Data Privacy Framework) - as offering "essentially equivalent" data protection. If your data is headed to one of these countries, you're in luck: no extra safeguards are needed, and the transfer is treated much like one within the EU. Since July 10, 2023, U.S. companies self-certifying under the Data Privacy Framework can receive EU patient data without requiring additional measures.
If there’s no adequacy decision, you’ll need Standard Contractual Clauses (SCCs). These pre-approved contracts, updated in June 2021, come in four modules (covering combinations like controller-to-controller or processor-to-processor) and don’t require prior approval from a Data Protection Authority. However, following the Schrems II ruling, you’re also obligated to perform a Transfer Impact Assessment (TIA) to confirm that the destination country’s legal system doesn’t undercut the protections guaranteed by SCCs. If risks are identified, you may need to implement extra safeguards.
For large multinational healthcare organizations frequently sharing data internally, Binding Corporate Rules (BCRs) can simplify things. These create a single, company-wide policy for global data transfers within the corporate group. While BCRs eliminate the need for individual SCCs between subsidiaries, they do require approval from a lead Data Protection Authority - a process that can take over a year.
Lastly, Derogations under Article 49 are available for rare, one-off transfers in exceptional situations. For instance, a transfer might be allowed if a patient gives explicit consent after being fully informed of the risks or if it’s necessary to protect someone’s life. However, these exceptions are not suitable for routine operations.
As Laura Bradford, Senior Research Associate at the University of Cambridge, puts it:
"SCCs provide an alternative, multi-layered standard for data protection that encompasses law, technology and organizational commitments."
Next, we’ll dive into how these mechanisms compare across key operational factors.
Comparison of Transfer Mechanisms
Each method comes with its own set of requirements and ideal use cases. Here’s a breakdown to help you choose the right approach:
| Mechanism | Requirements | Best For | Advantages | Disadvantages |
|---|---|---|---|---|
| Adequacy Decision | No additional safeguards beyond GDPR | Transfers to approved "safe" countries (e.g., UK, Switzerland, Japan) | No extra contracts or safeguards needed; easy data flow | Limited to specific countries on the approved list |
| Standard Contractual Clauses (SCCs) | Signed, unaltered clauses; TIA required | Routine transfers between unrelated entities | Widely accepted and easy to implement; no prior DPA approval needed | Requires a TIA for each destination, adding complexity |
| Binding Corporate Rules (BCRs) | DPA approval; legally binding internal framework | Multinational groups with frequent internal transfers | Simplifies all intra-group transfers with one policy | Expensive to implement and lengthy approval process (12+ months) |
| Derogations (Article 49) | Explicit patient consent or necessity test | Rare, one-off transfers (e.g., emergencies) | No formal contracts or approvals required | Extremely restrictive; unsuitable for routine use |
Before diving into SCCs, always check if the destination country has an adequacy decision to avoid unnecessary complications. For U.S. transfers, confirm that your partner is certified under the Data Privacy Framework - it can save a lot of time. Similarly, if you’re managing data transfers from both the EU and UK, use the EU SCCs alongside the UK Addendum to streamline compliance across both regions.
Data Privacy & Compliance: GDPR, HIPAA & Best Practices Explained
GDPR vs HIPAA: Cross-Border Healthcare Compliance
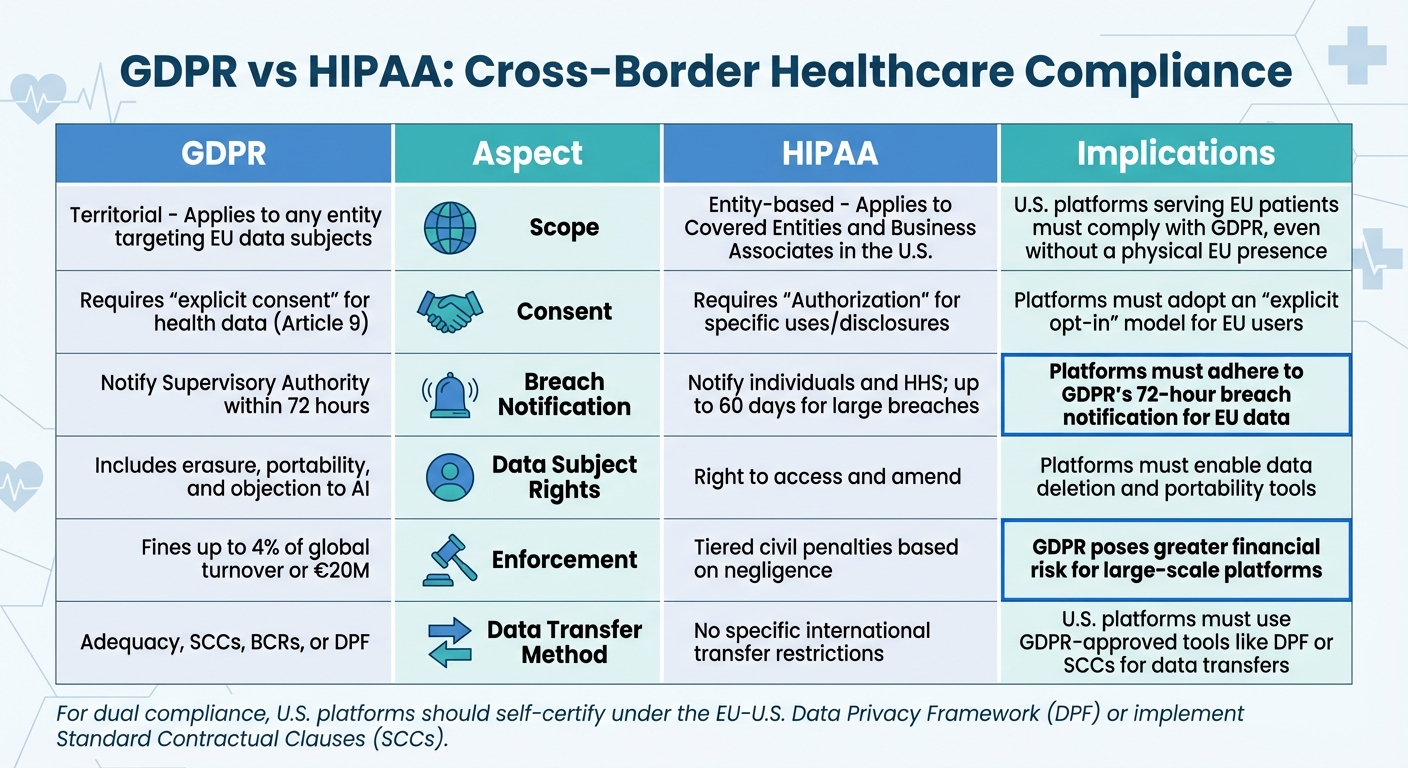
GDPR vs HIPAA: Key Compliance Differences for Cross-Border Healthcare Platforms
Key Differences and Overlaps
For U.S. platforms serving European patients, navigating the distinct frameworks of GDPR and HIPAA is no small task. GDPR is territorial, meaning it applies to any organization globally that processes the personal data of individuals in the EU. HIPAA, on the other hand, is entity-based, covering "Covered Entities" like healthcare providers, health plans, and clearinghouses, along with their "Business Associates" operating within the U.S. This means your platform could be subject to GDPR even if it doesn’t qualify as a HIPAA Covered Entity, simply by engaging with EU patients. This difference shapes how consent, breach protocols, and enforcement are handled.
Consent requirements under these frameworks vary greatly. GDPR demands explicit consent for processing health data under Article 9, which is a stricter standard than HIPAA's "Authorization." HIPAA often permits data use for treatment, payment, and healthcare operations without requiring specific patient consent. For platforms dealing with EU users, this means shifting from an opt-out model to an explicit opt-in system.
Timelines for breach notifications also differ. GDPR requires notification to the Supervisory Authority within 72 hours of a breach, while HIPAA allows up to 60 days for large breaches. If your platform handles both U.S. and EU patient data, the stricter 72-hour GDPR timeline becomes the de facto standard.
Financial penalties further highlight the differences. GDPR fines can reach up to €20,000,000 or 4% of a company's global annual revenue, whichever is higher. In contrast, HIPAA imposes tiered penalties based on the level of negligence. For large-scale platforms, the financial stakes under GDPR can be significantly higher. These distinctions underscore the operational complexities of managing compliance across both frameworks.
GDPR vs HIPAA Comparison Table
To better understand the distinctions, here’s a side-by-side comparison:
| Aspect | GDPR Details | HIPAA Details | Implications for Cross-Border Platforms |
|---|---|---|---|
| Scope | Territorial: Applies to any entity targeting EU data subjects | Entity-based: Applies to Covered Entities and Business Associates in the U.S. | U.S. platforms serving EU patients must comply with GDPR, even without a physical EU presence |
| Consent | Requires "explicit consent" for health data (Art. 9) | Requires "Authorization" for specific uses/disclosures | Platforms must adopt an "explicit opt-in" model for EU users |
| Breach Notification | Notify Supervisory Authority within 72 hours | Notify individuals and HHS; up to 60 days for large breaches | Platforms must adhere to GDPR's 72-hour breach notification for EU data |
| Data Subject Rights | Includes erasure, portability, and objection to AI | Right to access and amend | Platforms must enable data deletion and portability tools |
| Enforcement | Fines up to 4% of global turnover or €20M | Tiered civil penalties based on negligence | GDPR poses greater financial risk for large-scale platforms |
| Data Transfer Method | Adequacy, SCCs, BCRs, or DPF | No specific international transfer restrictions | U.S. platforms must use GDPR-approved tools like DPF or SCCs for data transfers |
For dual compliance, U.S. platforms need to self-certify under the EU-U.S. Data Privacy Framework (DPF), which the European Commission recognized on July 10, 2023. This framework simplifies data transfers by establishing adequacy, eliminating the need for additional safeguards like SCCs. If DPF certification isn’t an option, Standard Contractual Clauses (SCCs) are the next best solution. Just don’t forget to conduct a Transfer Impact Assessment to ensure U.S. laws don’t compromise GDPR protections.
Best Practices for GDPR Compliance in Patient Platforms
Data Mapping and Protection Measures
Getting GDPR compliance right starts with understanding how patient data flows within your organization. Start by documenting all data transfers, especially those leaving the European Economic Area (EEA). These could involve cloud storage providers, analytics platforms, or partner organizations. If the destination country lacks an adequacy decision, you'll need to implement Standard Contractual Clauses (SCCs) or Binding Corporate Rules (BCRs).
To safeguard patient data, encrypt it both at rest and during transit. Incorporate data protection by design from the outset. As required by Article 30, maintain detailed records of data categories and any international recipients. For each transfer, conduct a Transfer Impact Assessment (TIA) and apply data minimization principles - only transfer what's absolutely necessary.
Once you've securely mapped and protected data flows, the next step is ensuring patients' rights are respected and that breach notification protocols are robust.
Patient Rights and Breach Notification
GDPR gives patients significant control over their personal data. You must respond to their requests - like accessing, correcting, or erasing their data - within one month.
Streamline this process by setting up a secure Data Subject Request (DSR) portal. Use encrypted forms to allow patients to exercise their rights. When a patient requests data rectification or erasure, automate notifications to all data recipients and subprocessors to ensure compliance.
In the event of a data breach, GDPR mandates notifying the supervisory authority within 72 hours of detection, unless the breach is unlikely to pose a risk to individuals.
Implementing these practices effectively often requires leveraging specialized tools designed for GDPR compliance.
Using Compliance-Ready Tools like PatientPartner
Building a GDPR-compliant system from the ground up can be time-consuming and costly. That's where platforms like PatientPartner come in. Designed specifically for cross-border patient engagement, PatientPartner offers built-in compliance features to simplify data protection and consent management.
For pharmaceutical and med-tech companies running patient mentorship programs across different countries, PatientPartner eases the challenges of international data transfers. The platform adheres to SCCs and adequacy decisions, ensuring seamless compliance while connecting patients with mentors across jurisdictions. By combining technical safeguards with robust patient rights management, PatientPartner allows organizations to meet GDPR requirements without compromising operational efficiency. This enables healthcare companies to focus on improving patient adoption and treatment adherence strategies while staying compliant.
Conclusion
Adhering to GDPR regulations isn’t just about checking a box - it’s about fostering trust, particularly in cross-border patient engagement. Patients deserve to know their sensitive information is secure, no matter where it’s processed or stored. As the European Commission puts it, "The protection offered by the General Data Protection Regulation (GDPR) travels with the data, meaning that the rules protecting personal data continue to apply regardless of where the data lands".
For pharmaceutical and med-tech companies working on a global scale, compliance provides more than just legal assurance - it empowers patients and reinforces their confidence. Recent acknowledgments from the European Commission highlight that cross-border operations can meet stringent data protection requirements. By using approved data transfer mechanisms and conducting thorough impact assessments, healthcare organizations can maintain compliance while ensuring patient trust.
But the benefits of compliance go beyond legal frameworks. Strong data protection practices can directly enhance patient care. When patients feel secure about their data, they’re more likely to engage with support programs, share their experiences, and stick to their treatment plans. This trust creates an environment where mentorship programs and informed decision-making can thrive, ultimately leading to better outcomes.
Platforms like PatientPartner exemplify how integrating GDPR safeguards into strategies for enhancing patient engagement can simplify these complex requirements. By embedding compliance into the core of their infrastructure, healthcare organizations can focus on what truly matters - connecting patients with the resources and support they need. This approach not only ensures data security but also strengthens patient relationships, turning compliance into a powerful advantage in international healthcare.
FAQs
Does GDPR apply to U.S.-based platforms handling data from EU patients?
Yes, GDPR applies to platforms located outside the EU, including those in the U.S., if they handle personal data belonging to EU residents. This could involve offering services to EU patients or tracking their activities, such as gathering health-related data or connecting patients with mentors. U.S.-based companies must meet GDPR requirements, which include establishing a lawful basis for data processing, safeguarding data rights, integrating privacy-by-design principles, and implementing stringent security protocols.
When EU patient data is transferred to a platform outside the EU, it qualifies as a data export and must comply with approved mechanisms such as standard contractual clauses (SCCs) or binding corporate rules (BCRs). Companies are also required to evaluate transfer risks, use protective measures like encryption, and ensure ongoing alignment with GDPR regulations, even after the data has left the EU. For platforms like PatientPartner, incorporating these measures and preparing for potential regulatory scrutiny is critical when managing EU patient data.
What are the main differences between GDPR and HIPAA for cross-border healthcare platforms?
GDPR and HIPAA take distinct approaches to protecting health data, especially when it comes to cross-border situations. GDPR is a wide-reaching privacy regulation that applies to any organization handling the personal data of EU residents, regardless of the industry. It imposes strict rules on transferring data outside the European Economic Area (EEA) and grants individuals extensive rights, including the ability to access, correct, or delete their data. Additionally, organizations must establish a clear legal basis for every data processing activity they undertake.
In contrast, HIPAA is a U.S.-specific law designed to protect electronic Protected Health Information (ePHI) within the healthcare sector. It applies only to "covered entities" and their business associates, requiring adherence to Security and Privacy Rules, regular risk assessments, and Business Associate Agreements. Unlike GDPR, HIPAA does not provide individuals with broad rights or specify detailed procedures for international data transfers.
For platforms operating across borders, this means navigating GDPR's detailed requirements for data transfers and user rights for EU patients, while also ensuring compliance with HIPAA's security and contractual rules for U.S. patients.
What are the GDPR requirements for sharing patient data outside the EU?
To share patient data beyond the EU under GDPR, organizations need to establish strong safeguards to protect that information. These measures might involve legally binding agreements between public authorities, binding corporate rules, standard contractual clauses, or approved codes of conduct and certification mechanisms. On top of that, there must be enforceable rights for data subjects and effective legal remedies to ensure compliance with the regulations.
For digital health platforms, particularly those handling cross-border patient interactions, prioritizing these safeguards isn't just about following the law - it's about maintaining trust and meeting regulatory expectations.
Related Blog Posts
Author

Co-Founder and CEO of PatientPartner, a health technology platform that is creating a new type of patient experience for those going through surgery
Related Articles
Stay Ahead in Patient Engagement
We and selected third parties use cookies or similar technologies for technical purposes and, with your consent, for other purposes as specified in the cookie policy. Use the “Accept” button or close this notice to consent.
We and selected third parties use cookies or similar technologies for technical purposes and, with your consent, for other purposes as specified in the cookie policy. Use the “Accept” button or close this notice to consent.
We and selected third parties use cookies or similar technologies for technical purposes and, with your consent, for other purposes as specified in the cookie policy. Use the “Accept” button or close this notice to consent.






